ConvergeHEALTH Safety™ : Shaping the future of pharmacovigilance
2016 - 2017 | 2 Designers
THE CHALLENGE
Life sciences organizations must continually manage and report adverse events to regulatory bodies (known as Pharmacovigilance). For a long time, the antiquated systems made this difficult and created compliance risks. The industry was in dire need of a better solution.
On this project, I collaborated with another designer and focused on experience decisions and execution, culminating in the launch of ConvergeHEALTH Safety™ to two major pharma companies with multi-million-dollar contracts.
PROJECT GOAL
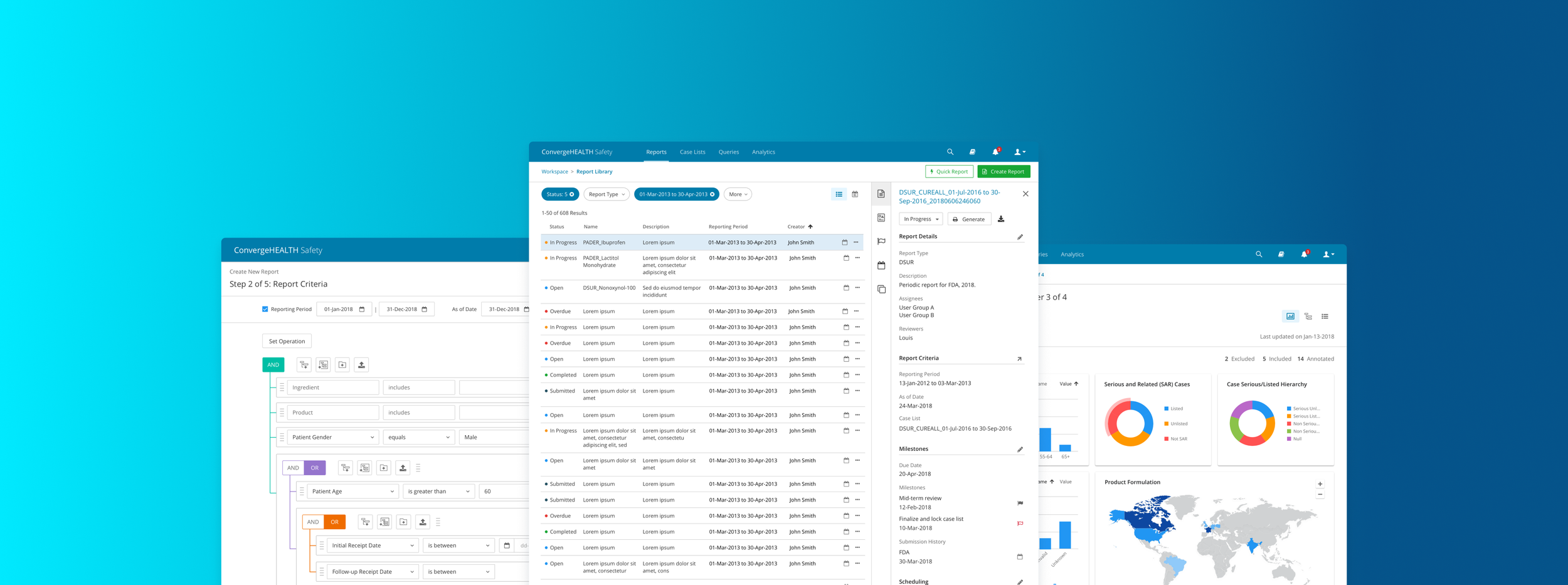
Design a modern, scalable application to help Safety Analysts and Safety Managers track adverse events, query data and build regulatory reports.
USER RESEARCH
We conducted in-depth interviews and collab sessions with clinical research associates (CRA), safety analysts and Deloitte practitioners in the industry.
IA & Early ideation
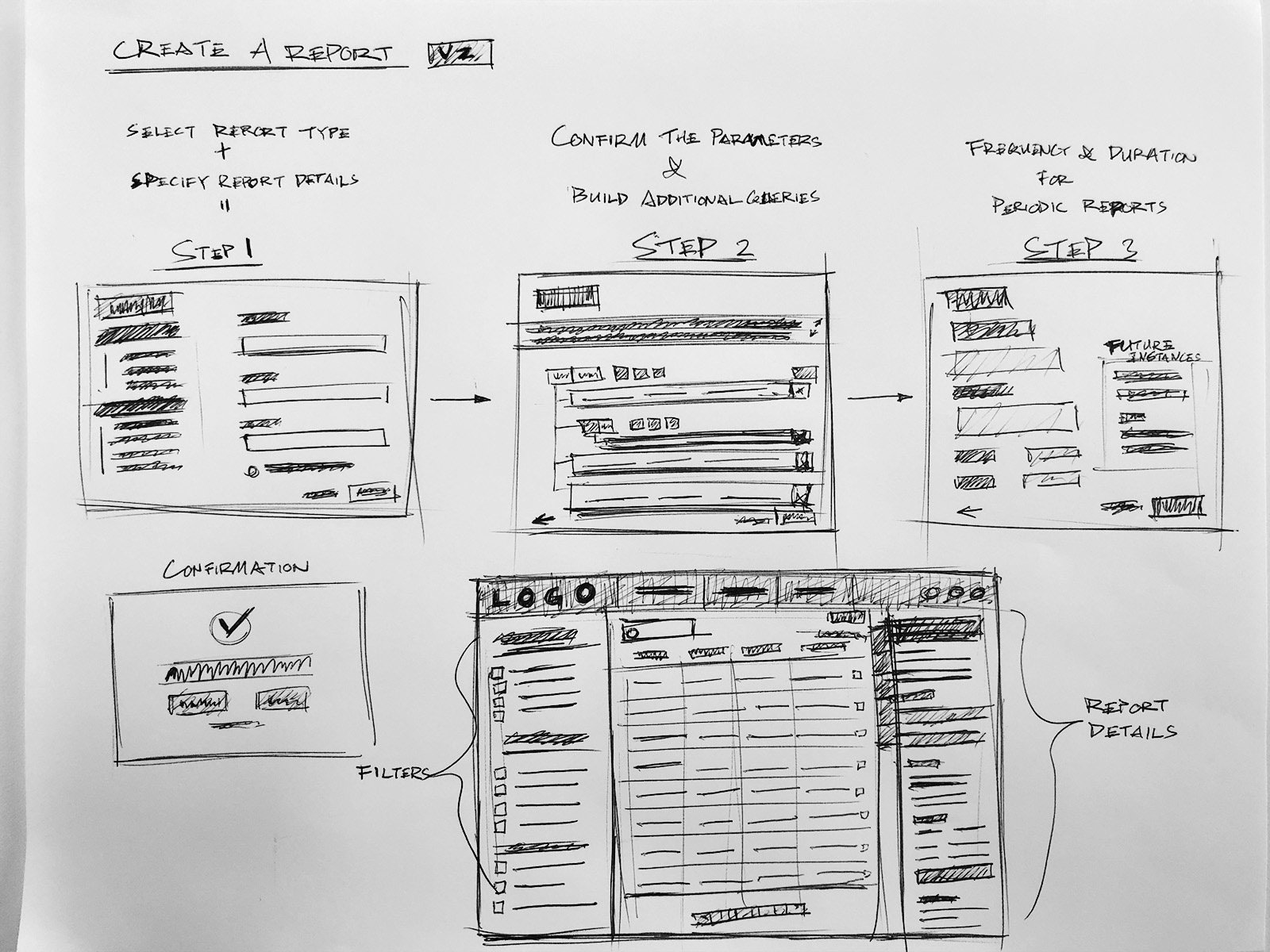
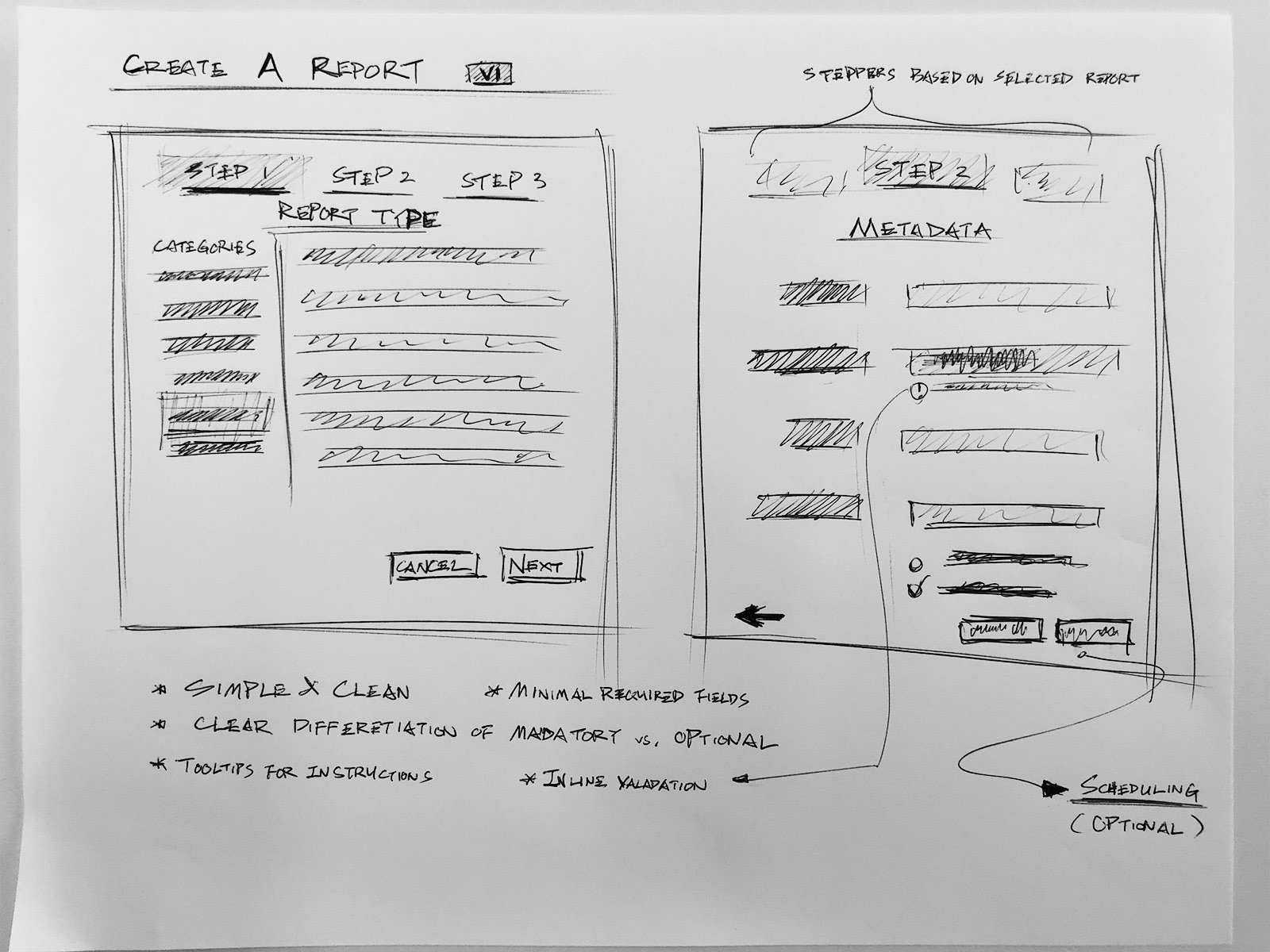
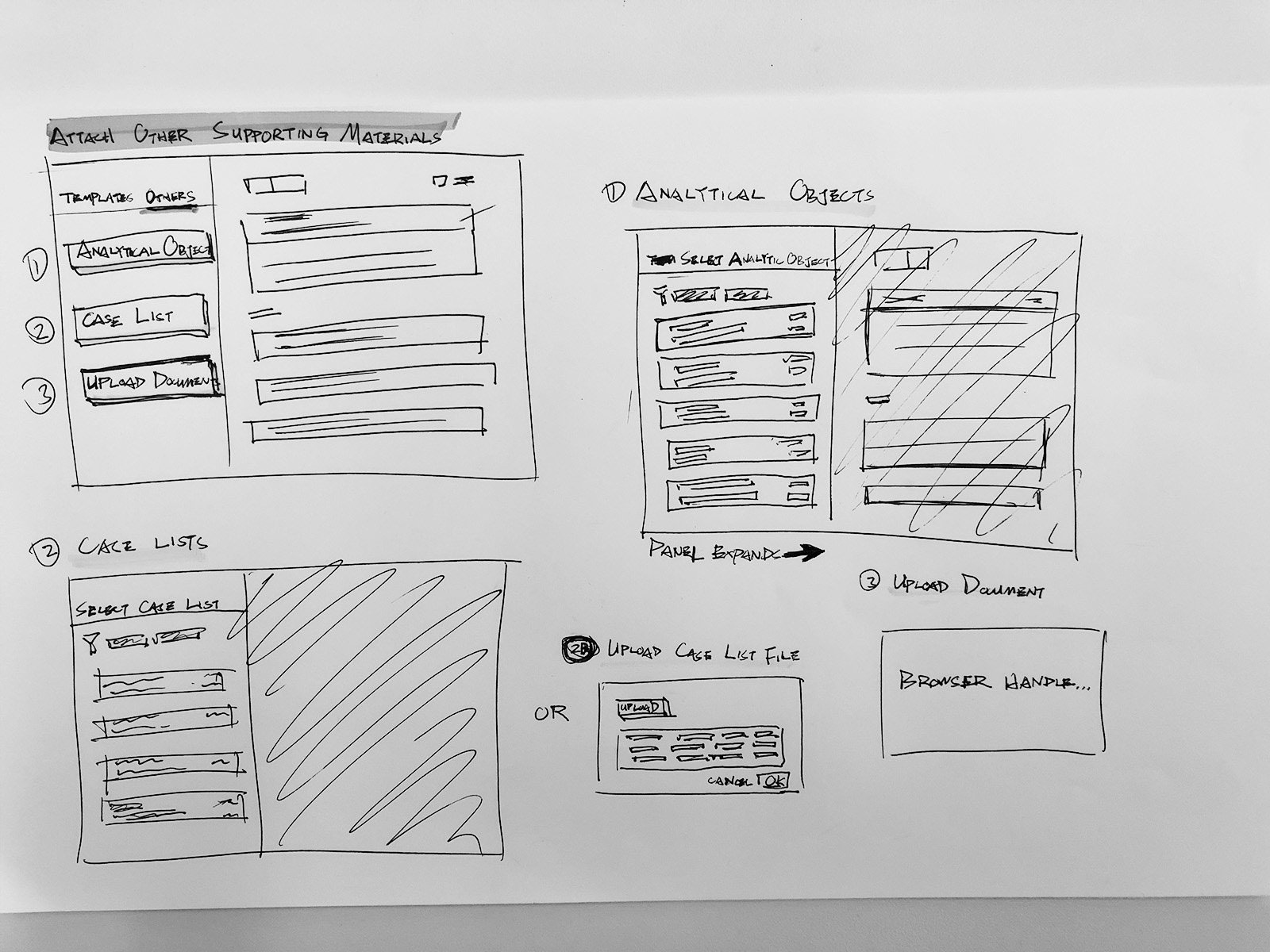

Intuitive query builder to compile cases
The “Visual Story” to break down complex case data
Test and iterate to optimize content filtering
IMPACT
I designed and shipped the very first release of ConvergeHEALTH Safety, its simplified UX and unified BI capability was endorsed by safety analysts pilot users, which played a significant role in the product being adopted by two major pharmaceutical clients (multi-million dollar contracts) , and became one of the biggest product offering that graduated from the Deloitte iLab incubator. ConvergeHEALTH is now a billion-dollar business. Read more on the latest of CH Safety suite here.




